
How to Choose Between Lithium Metal vs Lithium Ion?
Are you struggling to select the right battery technology for your application? Many engineers and procurement managers face confusion when comparing lithium metal vs lithium ion batteries.
Lithium metal batteries use pure lithium anodes and offer higher energy density for single-use applications, while lithium ion batteries use lithium compounds with graphite anodes and provide rechargeable capabilities.
The choice depends on your specific needs: lithium metal batteries excel in long-term, low-drain devices like industrial meters, whereas lithium ion batteries suit applications requiring frequent recharge cycles like consumer electronics.

The wrong choice can lead to reduced performance, safety risks, and wasted investment in unsuitable power solutions.
At Long Sing Industrial, we’ve spent over a decade navigating the fine line between these two chemistries to serve sectors where “standard” off-the-shelf power is never enough.
The Long Sing Experience: Solving the “Pulse” Gap
In my years leading technical integration at Long Sing Industrial, I’ve seen countless engineers struggle with the “High Capacity vs. High Power” dilemma.
One of our most significant breakthroughs involved a major Smart Water Meter manufacturer.
They needed a battery that could last 10 years (Lithium Metal territory) but required high-current pulses for 5G communication (Lithium Ion territory).
Instead of forcing a choice between the two, we developed the Hybrid Pulse Capacitor (HPC) System.
- The Core: We utilize a high-capacity Lithium Thionyl Chloride (Li-SOCl2) cell to provide the long-term energy reservoir.
- The “Booster”: We parallel it with our proprietary HPC.
- The Result: The HPC acts as a buffer, storing energy from the metal battery and releasing it in 2A–5A bursts for data transmission, even in extreme temperatures (-40°C to +85°C).
By combining the energy density[1] of Lithium Metal with the power delivery characteristics of Lithium Ion systems, we eliminated the voltage delay that typically kills smart devices.
This isn’t just theory; it’s a solution we’ve refined through millions of units deployed in the field, earning us recognitions like the CES Innovation Award and the Edison Silver Award.
Want to try our long life lithium batteries?
Submit the form below, and Long Sing Industrial engineers will analyze your power profile for free.
This guide below will walk you through the key differences and help you make an informed decision based on technical specifications, application requirements, and cost considerations.
Table of Contents
- What Are the Core Differences Between Lithium Metal and Lithium Ion Batteries?
- Which Battery Type Offers Better Energy Density?
- How Do Safety Considerations Differ Between These Technologies?
- What Are the Cost Implications Over the Battery Lifecycle?
- Which Applications Benefit Most from Each Technology?
What Are the Core Differences Between Lithium Metal and Lithium Ion Batteries?
Lithium metal batteries utilize pure metallic lithium as the anode material and are typically non-rechargeable primary cells, while lithium ion batteries employ lithium compounds intercalated in graphite anodes and function as rechargeable secondary cells.

The fundamental distinction lies in their electrochemical structure[2]:
Lithium metal batteries achieve direct oxidation-reduction reactions, whereas lithium ion batteries rely on lithium ion movement between electrodes.
Understanding the Electrochemical Architecture
The lithium metal battery vs lithium ion debate starts at the molecular level.
Lithium metal batteries contain a pure lithium anode that undergoes irreversible oxidation during discharge. This process releases electrons directly from the metallic lithium, creating a high voltage potential.
The cathode materials commonly include manganese dioxide, carbon monofluoride, or sulfur dioxide. These batteries operate through a one-way chemical reaction that cannot be reversed efficiently.
Lithium ion batteries work differently. They store energy through a reversible intercalation process.
During charging, lithium ions move from the cathode through an electrolyte to insert themselves between graphite layers in the anode. During discharge, these ions travel back to the cathode.
This back-and-forth movement allows hundreds or thousands of charge cycles. The most common cathode chemistries include lithium cobalt oxide, lithium iron phosphate, and lithium nickel manganese cobalt oxide.
The electrolyte composition also differs significantly. Lithium metal batteries often use non-aqueous organic solvents with lithium salts, and some specialized variants like lithium thionyl chloride batteries employ liquid cathodes.
Lithium ion batteries typically use lithium hexafluorophosphate dissolved in organic carbonates, which facilitates ion transport while maintaining stability across multiple cycles.
Structural Components Comparison
| Component | Lithium Metal Battery | Lithium Ion Battery |
|---|---|---|
| Anode Material | Pure metallic lithium | Graphite with lithium compounds |
| Cathode Material | MnO2, CFx, SOCl2, SO2 | LiCoO2, LiFePO4, NMC |
| Electrolyte | Organic solvent with Li salts | LiPF6 in organic carbonates |
| Separator | Porous polymer or glass fiber | Polyethylene or polypropylene |
| Rechargeability | Primary (non-rechargeable) | Secondary (rechargeable) |
| Typical Voltage | 3.0-3.6V | 3.6-3.7V |
The physical construction reflects these chemical differences.
Lithium metal batteries can be designed with thinner profiles because they don’t need to accommodate repeated expansion and contraction. The pure lithium anode provides maximum energy storage in minimal space.
Lithium ion batteries require more robust construction to handle mechanical stress from charging cycles.
The graphite anode expands and contracts as lithium ions intercalate and deintercalate, requiring careful engineering to prevent structural degradation.
Manufacturing processes also diverge significantly.
Lithium metal battery production involves handling highly reactive metallic lithium in controlled atmospheres, typically argon or dry rooms with extremely low humidity.
The assembly must prevent any contact between lithium and moisture, which would cause immediate oxidation.
Lithium ion battery manufacturing focuses on precise coating of electrode materials, controlled drying processes, and formation cycling to establish stable solid electrolyte interphase layers.
These different manufacturing approaches affect production costs, quality control requirements, and scaling possibilities.
Which Battery Type Offers Better Energy Density?
Lithium metal batteries deliver superior gravimetric energy density, typically ranging from 300-500 Wh/kg, compared to lithium ion batteries which offer 150-250 Wh/kg.

This advantage stems from lithium metal’s theoretical specific capacity of 3,860 mAh/g versus graphite’s 372 mAh/g in lithium ion cells.
However, lithium ion batteries can achieve comparable volumetric energy density[3] through optimized cell design and packaging.
Gravimetric vs Volumetric Energy Considerations
When comparing lithium metal vs lithium ion batteries on energy density, we must consider both weight and volume metrics.
Gravimetric energy density measures watt-hours per kilogram, which matters most for weight-sensitive applications like aerospace, portable medical devices, and remote sensors.
Volumetric energy density measures watt-hours per liter, which becomes critical when space constraints dominate design decisions.
Lithium metal batteries excel in gravimetric energy density because metallic lithium is the lightest solid element and has the highest electrochemical potential.
A typical lithium thionyl chloride cell achieves 350-500 Wh/kg, making it ideal for applications where weight reduction directly translates to performance benefits.
These batteries can power remote monitoring equipment for 10-20 years without replacement, thanks to their exceptional energy storage capabilities combined with low self-discharge rates[4].
Lithium ion batteries have improved dramatically over the past two decades. Modern lithium ion cells reach 200-250 Wh/kg through advanced cathode materials and thinner separators.
While this falls short of lithium metal battery performance, lithium ion technology compensates through rechargeability.
A single lithium ion battery can replace hundreds of disposable lithium metal batteries over its lifetime, which changes the energy density calculation when considering total energy delivered per unit mass across the battery’s service life.
Real World Energy Density Performance Comparison
| Performance Metric | Lithium Metal Battery | Lithium Ion Battery | Impact on Application |
|---|---|---|---|
| Gravimetric Energy Density | 300-500 Wh/kg | 150-250 Wh/kg | Critical for weight-sensitive devices |
| Volumetric Energy Density | 600-800 Wh/L | 400-700 Wh/L | Important for space-constrained designs |
| Self-Discharge Rate | <1% per year | 2-5% per month | Affects long-term storage capacity |
| Operating Temperature | -55°C to +85°C | -20°C to +60°C | Determines environmental suitability |
| Cycle Life | Single use | 500-3000 cycles | Defines replacement frequency |
| Discharge Rate Capability | Low to moderate | Moderate to very high | Limits power-intensive applications |
The discharge rate capability significantly impacts practical energy delivery.
Standard lithium metal batteries perform best at low to moderate discharge rates, typically below 1C. This makes them perfect for applications with steady, predictable power demands like utility meters and remote sensors.
When pulse power is needed, manufacturers like Long Sing Industrial have developed hybrid pulse capacitor solutions that combine lithium primary cells with supercapacitors to handle brief high-current demands while maintaining the energy density advantages of lithium metal chemistry.
Lithium ion batteries can deliver much higher discharge rates, often 5C to 10C for power-optimized cells, and some specialized designs reach 20C or higher.
This capability makes the lithium ion vs lithium metal decision clear for applications like power tools, electric vehicles, and grid storage systems where rapid energy discharge is essential.
The trade-off is that high-rate lithium ion cells sacrifice some energy density to achieve their power output capabilities.
Temperature performance also affects usable energy density.
Lithium metal batteries maintain most of their capacity across a wide temperature range, from -55°C to +85°C, though discharge rate capability decreases at temperature extremes.
Lithium ion batteries show more sensitivity to temperature, with significant capacity loss below -20°C and accelerated degradation above 45°C.
This temperature sensitivity means that lithium metal batteries deliver their rated energy density more reliably across diverse environmental conditions, while lithium ion batteries may require thermal management systems that add weight and volume.
How Do Safety Considerations Differ Between These Technologies?
Lithium metal batteries present higher inherent reactivity risks due to pure metallic lithium’s tendency to form dendrites and react violently with water, but their non-rechargeable nature eliminates overcharge hazards.
Lithium ion batteries carry thermal runaway risks from internal short circuits and overcharging, yet decades of safety engineering have produced robust protection systems.

Both technologies require specific handling protocols, with lithium metal batteries demanding stricter moisture control and lithium ion batteries needing sophisticated battery management systems[5].
Chemical Reactivity and Failure Modes
The safety profile when comparing lithium metal battery vs lithium ion technology begins with fundamental chemical properties.
Metallic lithium reacts vigorously with water, producing lithium hydroxide and hydrogen gas. Even atmospheric moisture can cause surface oxidation, creating heat and potentially igniting the hydrogen byproduct.
This reactivity requires manufacturers to assemble lithium metal batteries in extremely dry environments, typically with dew points below -60°C.
Once sealed properly, lithium metal batteries remain stable for decades, but any breach of the cell casing can expose reactive lithium to ambient moisture.
Lithium ion batteries contain no metallic lithium in their charged or discharged state.
The lithium exists only as ions within compound structures. This makes them less reactive to moisture and air exposure.
However, lithium ion batteries face different safety challenges. When damaged or overcharged, the organic electrolyte can decompose and release flammable gases.
If internal temperatures rise above 150°C, the cathode materials can decompose and release oxygen, which feeds combustion of the organic electrolyte.
This thermal runaway process can cause fires or explosions in severe cases.
Dendrite formation represents a critical safety concern for lithium metal chemistry.
During discharge, metallic lithium can deposit unevenly, forming needle-like structures called dendrites.
If dendrites grow large enough, they can pierce the separator and create an internal short circuit. This risk is one reason why lithium metal batteries remain primarily non-rechargeable.
Attempts to recharge lithium metal batteries multiply dendrite formation[6] risks and increase the likelihood of catastrophic failure.
Lithium ion batteries avoid this issue because lithium ions intercalate into the graphite structure rather than plating as metal.
Safety Systems and Protection Mechanisms
| Safety Feature | Lithium Metal Battery | Lithium Ion Battery |
|---|---|---|
| Overcharge Protection | Not applicable (non-rechargeable) | Essential: voltage cutoff circuits |
| Over-discharge Protection | Built-in voltage depression | Required: undervoltage lockout |
| Temperature Monitoring | Optional for harsh environments | Critical: thermistors and cutoffs |
| Current Limiting | Internal resistance provides limiting | Active circuits with MOSFETs |
| Pressure Relief | Vent mechanisms for gas buildup | Current interrupt devices (CIDs) |
| Cell Balancing | Not needed (single use) | Required for multi-cell packs |
| Shipping Classification | UN3090/UN3091 restrictions | UN3480/UN3481 with testing |
Lithium metal batteries incorporate passive safety features. The battery’s inherent chemistry provides some protection.
For example, lithium manganese dioxide batteries form a protective passivation layer on the lithium anode surface, which limits self-discharge and reduces reactivity.
Manufacturers design these cells with pressure relief vents that release gases safely if internal pressure builds due to abuse or manufacturing defects.
The hermetic sealing prevents moisture ingress under normal conditions, and the non-rechargeable design eliminates risks associated with charging systems.
Lithium ion batteries require active safety management.
Modern lithium ion packs include sophisticated battery management systems with multiple protection layers. These systems monitor cell voltage[7], temperature, and current in real-time.
If any parameter exceeds safe limits, the BMS can disconnect the battery from the load or charger.
Positive temperature coefficient devices increase resistance as temperature rises, providing automatic current limiting during fault conditions.
Current interrupt devices permanently disconnect the cell if internal pressure exceeds design limits, preventing rupture.
Transportation regulations reflect these safety differences. Both battery types face restrictions under international dangerous goods regulations, but the specific requirements vary.
Lithium metal batteries have stricter lithium content limits per cell and per package.
Airlines often impose more restrictive limits on lithium metal battery shipments compared to lithium ion.
This affects logistics costs and delivery times for devices using these batteries.
So how can you ship the battery products safely?
Safety & Risks: What You Need to Know before Shipping
Shipping safety is not just a regulatory checkbox—it is a responsibility to your brand, your customers, and the logistics chain that connects us.
At Long Sing Industrial, we manufacture both lithium batteries and lithium-ion batteries, and we see firsthand how misunderstandings about chemistry and packaging can cause serious problems in transit.
In the debate of lithium metal vs lithium ion, lithium metal cells contain reactive metallic lithium, while lithium-ion cells store lithium in an intercalated form inside the electrodes. This difference drives how they are classified, packed, and declared when shipped by air, sea, or road.
Buyers who only think in terms of performance often underestimate how critical lithium vs lithium ion is for logistics compliance.
Lithium metal batteries are assigned stricter UN codes and quantity limits because of their higher fire and short-circuit risk.
A lithium ion battery vs lithium battery shipment usually allows higher watt-hour limits per package, but only if the cells have passed UN38.3 testing and are packed to prevent movement and terminal contact. Mixing products without proper documentation is one of the most common reasons we see containers stopped at ports.
Procurement teams also need to think beyond labels. Whether you are shipping a lithium battery vs lithium ion battery for energy storage, industrial tools, or mobility systems, the packaging, state of charge, and pallet configuration must match the chemistry.
In projects that involve lithium and lithium ion batteries in the same order, we always separate them into different cartons with independent declarations to reduce compliance risk.
Ultimately, choosing between lithium metal vs lithium ion is not only about cost and energy density—it is about safe, predictable global delivery. As a factory, our role is to guide you through regulations, testing, and packaging so your batteries arrive safely, legally, and on schedule, protecting both your investment and your reputation.
The practical safety record also matters when evaluating lithium metal battery vs lithium ion battery choices.
Lithium ion batteries have experienced high-profile safety incidents, including smartphone recalls and aircraft fires.
However, these incidents affected a tiny fraction of the billions of lithium ion cells produced annually. The failure rate for quality lithium ion batteries is less than 1 in 10 million cells.
Lithium metal batteries have an excellent safety record in their intended applications, with failures primarily resulting from mechanical damage, manufacturing defects, or misuse rather than inherent chemistry instabilities.
When installed in inaccessible locations like underground utility meters, their long-term stability without maintenance provides substantial safety advantages.
What Are the Cost Implications Over the Battery Lifecycle?
Initial purchase costs favor lithium ion batteries at $150-300 per kWh versus $800-1500 per kWh for lithium metal batteries, but total cost of ownership depends on application duration, replacement frequency, and maintenance access.
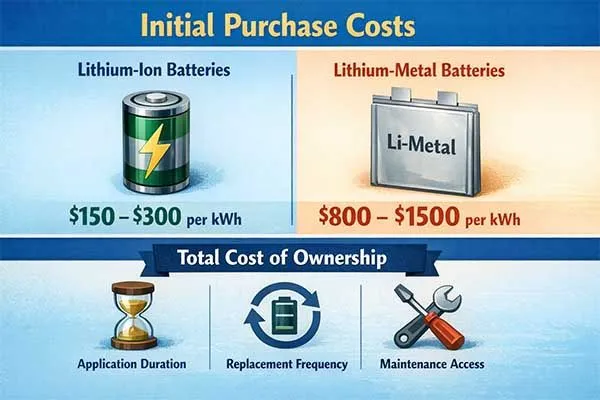
Lithium metal batteries eliminate replacement costs for 10-20 year deployments in inaccessible locations, while lithium ion batteries offer lower amortized costs for accessible applications with shorter service intervals through their rechargeability.
Upfront Investment Analysis
The cost comparison between lithium ion and lithium metal batteries starts with unit pricing.
Lithium ion cells benefit from massive production volumes driven by consumer electronics and electric vehicle markets.
Gigafactory-scale production has driven costs down dramatically, with quality 18650 cells available for under $2 per cell and large-format prismatic cells reaching $150 per kWh.
This economy of scale makes lithium ion attractive for any application where the initial battery cost significantly impacts product pricing.
Lithium metal batteries cost substantially more per kilowatt-hour.
Specialized chemistries like lithium thionyl chloride require careful manufacturing in controlled environments with expensive raw materials.
Production volumes remain orders of magnitude lower than lithium ion, preventing similar cost reductions.
A single D-size lithium thionyl chloride cell might cost $15-30 in moderate quantities, compared to $3-5 for a similar capacity lithium ion cell.
This price differential means that projects with tight initial budgets often default to lithium ion even when lithium metal might provide better long-term value.
However, the purchase price tells only part of the financial story. System integration costs differ significantly between these technologies.
Lithium ion batteries require battery management systems, charging circuits, and often thermal management hardware.
These ancillary components can add $50-200 per device depending on complexity and safety requirements.
The device must include provisions for battery replacement[9] or recharging, which adds design complexity.
Lithium metal batteries need minimal support circuitry, typically just a voltage supervisor or low-battery indicator. The simpler system design reduces engineering costs, component counts, and assembly time.
Total Cost of Ownership Calculations
| Cost Factor | Lithium Metal Battery | Lithium Ion Battery | 10-Year Impact |
|---|---|---|---|
| Initial Battery Cost | $25-50 per unit | $10-20 per unit | Higher upfront for lithium metal |
| Support Electronics | $5-10 per unit | $50-150 per unit | Major savings with lithium metal |
| Installation Labor | $50-100 (one time) | $50-100 (one time) | Similar initial installation |
| Replacement Frequency | 0-1 times | 3-5 times | Fewer service calls for lithium metal |
| Service Visit Cost | $0-100 total | $300-500 total | Significant savings in remote locations |
| Disposal/Recycling | $2-5 per unit | $3-8 per replacement | Lower total disposal costs |
| Total 10-Year Cost | $80-165 per unit | $360-670 per unit | Lithium metal wins for remote devices |
The replacement and maintenance costs dramatically shift the economic calculation.
For devices installed in accessible locations like consumer electronics or building systems, lithium ion’s rechargeability provides clear cost advantages.
The user simply plugs in the device or swaps a standardized battery pack. Even if a lithium ion battery needs replacement after 2-3 years, the minimal labor cost and low battery price keep total ownership costs manageable.
In these scenarios, paying 3-5 times more for a lithium metal battery that might last 10-15 years makes little economic sense.
The equation reverses for remote or inaccessible installations.
Consider a utility meter on a pipeline in rural areas, a weather station on a mountaintop, or a seismic monitor in an underground location.
Each service visit requires a technician to travel to the site, access the device, replace the battery, and test the system.
These service calls can cost $200-500 per visit for remote locations, or much more for offshore or hazardous environments.
When you multiply this cost by 3-5 replacement cycles over 10-15 years, the total maintenance expense far exceeds any savings from cheaper lithium ion batteries.
Companies like Long Sing Industrial specialize in optimizing battery solutions for these long-life applications.
By selecting appropriate lithium metal chemistries and designing robust battery packs, they help customers eliminate maintenance costs over extended deployment periods.
The higher initial battery investment pays for itself within the first avoided service call, and every subsequent year provides additional cost savings compared to rechargeable alternatives.
Risk costs also factor into total ownership calculations.
Battery failure in critical applications can trigger expensive consequences.
A failed battery in a gas meter might require an emergency service call, cause service interruptions, or create safety hazards.
The ultra-low failure rates of quality lithium metal batteries reduce these risk costs.
Lithium ion batteries, while reliable, degrade predictably over time. The capacity fade and increased internal resistance mean that lithium ion batteries must be replaced before complete failure to maintain system reliability.
This forced replacement schedule adds cost even when the battery might still provide some functionality.
Which Applications Benefit Most from Each Technology?
Lithium metal batteries excel in long-term, low-drain applications including utility metering, industrial sensors, medical implants, and backup power systems where 10-20 year operational life without maintenance justifies higher upfront costs.

Lithium ion batteries dominate applications requiring frequent recharging such as consumer electronics, electric vehicles, power tools, and renewable energy storage where cycle life and power density outweigh per-cycle costs.
Industrial and Utility Applications
The lithium metal vs lithium ion battery decision becomes straightforward when examining specific application requirements.
Utility metering represents an ideal use case for lithium metal technology. Water, gas, and electric meters operate continuously with low, steady power draws.
Modern smart meters transmit data periodically, creating brief current pulses on top of the baseline consumption.
These meters are typically installed in locations where access requires scheduling, coordination with property owners, and sometimes specialized equipment.
A 20-year battery life matches typical meter replacement cycles and eliminates interim maintenance.
Lithium thionyl chloride batteries serve these applications exceptionally well, providing stable voltage output across their entire discharge curve and maintaining functionality from -55°C to +85°C.
The ultra-low self-discharge rate ensures that stored energy remains available throughout the battery’s lifetime.
For applications requiring occasional high-current pulses, manufacturers combine lithium primary cells with hybrid pulse capacitors, creating a system that delivers both long life and burst power capability.
Industrial sensors and monitoring equipment similarly benefit from lithium metal batteries.
Oil and gas pipeline sensors, environmental monitoring stations, and structural health monitoring systems often operate in remote or hazardous locations. These devices typically measure parameters periodically and transmit data wirelessly.
The combination of low average power consumption and infrequent maintenance access makes lithium metal batteries the clear choice.
The ability to operate across extreme temperature ranges[10] without performance degradation adds reliability in harsh industrial environments.
Safety and security systems represent another natural fit for lithium metal technology.
Fire alarm panels, emergency lighting systems, and security sensors require guaranteed operation for years without attention. The low self-discharge rate ensures readiness during emergencies.
Smoke detectors in commercial buildings might use lithium metal batteries to achieve 10-year sealed unit designs, eliminating the need for frequent battery replacements that often go ignored.
These applications prioritize reliability and long-term performance over any cost considerations.
Application-Specific Technology Selection
| Application Category | Preferred Technology | Key Decision Factors | Typical Battery Life |
|---|---|---|---|
| Smart Utility Meters | Lithium Metal | Remote installation, low power, long life | 15-20 years |
| Medical Implants | Lithium Metal | Biocompatibility, reliability, size | 5-15 years |
| Smartphones | Lithium Ion | Daily charging, size, cost | 2-3 years (500+ cycles) |
| Electric Vehicles | Lithium Ion | Rechargeability, power output, cost | 8-10 years (2000+ cycles) |
| Remote Sensors | Lithium Metal | Inaccessible location, low maintenance | 10-20 years |
| Power Tools | Lithium Ion | High power, frequent use, cost | 3-5 years (1000+ cycles) |
| Emergency Backup | Lithium Metal | Shelf life, reliability, readiness | 10-15 years |
| Grid Energy Storage | Lithium Ion | Daily cycling, scalability, economics | 10-15 years (4000+ cycles) |
Consumer electronics overwhelmingly favor lithium ion technology. Smartphones, laptops, tablets, and wearable devices all require daily or weekly recharging to support their high power consumption.
Users expect to charge these devices routinely, making rechargeability essential rather than optional. The compact form factors and high discharge rates needed for these applications align perfectly with lithium ion capabilities.
The lower cost per unit also matters in consumer markets where price sensitivity drives purchasing decisions.
The question of lithium metal batteries vs lithium ion batteries for these applications has been decisively answered by market forces.
Electric vehicles represent the largest growing market for lithium ion batteries. The ability to recharge quickly and deliver high power output for acceleration makes lithium ion the only viable option for automotive applications.
A typical EV battery pack undergoes one charge-discharge cycle per day and must support hundreds of kilowatts of power output.
Even though lithium ion batteries cost thousands of dollars per vehicle, the ability to recharge them thousands of times makes the per-mile cost acceptable.
Advances in fast-charging technology and increasing cycle life continue to improve the value proposition for EV batteries.
Power tools and garden equipment have transitioned rapidly from nickel-cadmium to lithium ion batteries.
Professional contractors demand the high power output for driving screws, cutting materials, or trimming hedges. The ability to quickly swap standardized battery packs between tools adds convenience.
Though individual battery packs cost $50-150, their reusability across multiple tools and hundreds of charge cycles provides acceptable economics for both professional and consumer users.
When considering lithium ion and lithium metal batteries for these applications, the rechargeability requirement eliminates lithium metal from consideration.
Renewable energy storage systems increasingly rely on lithium ion technology.
Solar and wind installations need batteries that can charge and discharge daily as renewable generation varies.
These systems might cycle once or twice daily for 10-15 years, requiring batteries that can handle 5,000+ cycles.
Lithium iron phosphate chemistry has become popular for these applications due to its excellent cycle life and thermal stability.
The decreasing cost of lithium ion batteries has made renewable energy storage economically viable for both utility-scale and residential installations, applications where lithium metal battery vs lithium ion battery selection clearly favors the rechargeable option.
Conclusion
Choosing between lithium metal vs lithium ion batteries requires careful analysis of your specific application requirements.
Lithium metal batteries excel in scenarios demanding ultra-long service life, minimal maintenance, and operation across extreme temperatures, particularly when devices are installed in remote or inaccessible locations.
Their superior energy density and exceptional shelf life make them ideal for utility metering, industrial sensors, and backup power systems where the higher initial cost is offset by elimination of replacement expenses.
Conversely, lithium ion batteries dominate applications requiring frequent recharge cycles, high power output, and cost-effective energy storage for accessible devices.
The decision ultimately depends on balancing upfront costs against total lifecycle expenses, considering operational requirements like discharge rates and temperature ranges, and evaluating the practical feasibility of battery replacement or recharging.
By understanding the fundamental differences between these technologies and honestly assessing your application’s needs, you can select the battery solution that delivers optimal performance, reliability, and value throughout your product’s lifetime.
Quick Lithium Manganese Dioxide Batteries FAQ Review(Click to Unfold)
Q: What is lithium metal?
A: Lithium metal is elemental lithium in metallic form. In batteries, “lithium metal” usually refers to cells that use metallic lithium as the anode, which are typically primary (non-rechargeable).
Q: Is lithium a metal?
A: Yes. Lithium is a chemical element and an alkali metal on the periodic table.
Q: How do I know if a battery is lithium-ion or lithium metal?
A: Check the label and specs: Li-ion cells are rechargeable and often marked “Li-ion/Li-poly” with a nominal 3.6–3.7V (or packs like 7.4V, 11.1V).
Lithium metal primary cells are usually marked CR/BR/FR (coin/cylindrical types) and are not rechargeable; coin cells are commonly 3.0V and marked “Lithium.”
Q: Is there a difference between lithium-ion and lithium metal batteries?
A: Yes. Lithium-ion batteries are rechargeable and require controlled charging and protection circuitry.
Lithium metal batteries are primary (non-rechargeable) and use metallic lithium, offering very low self-discharge and long shelf life, but must never be charged.
Q: Is a CR2032 battery a lithium-ion or metal battery?
A: A CR2032 is a lithium metal (primary) coin cell, not lithium-ion. It is typically 3.0V and non-rechargeable.
Q: Do lithium batteries contain lithium metal?
A: Some do. Lithium metal primary batteries contain metallic lithium, while lithium-ion batteries typically do not contain metallic lithium (they use lithium compounds and move lithium as ions during charge/discharge).
Q: Is a lithium metal battery safe?
A: It can be safe when used correctly (right device, correct polarity, no short-circuit, no charging).
Risks increase if the cell is shorted, overheated, damaged, or improperly recharged—lithium metal cells should never be recharged unless explicitly designed as rechargeable (rare/specialized chemistries).
Q: What is the safest type of lithium battery?
A: There’s no single “safest” for every use, but LiFePO₄ (LFP) lithium-ion is widely regarded as among the most thermally stable rechargeable lithium chemistries.
For non-rechargeable needs, many lithium metal primary cells are very safe in low-drain applications when properly handled and protected from shorts.
Q: What is the biggest cause of lithium-ion batteries exploding?
A: The most common root cause is thermal runaway triggered by abuse or defects—especially overcharging, internal short circuits (damage/manufacturing flaws), external shorting, or overheating. Proper protection circuits, correct chargers, and quality cells greatly reduce risk.
Q:Are lithium batteries the same as lithium-ion batteries?
A:No, they are not the same. Lithium batteries (primary batteries) feature metallic lithium as an anode and are designed for single use only. Lithium-ion batteries (secondary batteries) use lithium compounds in their chemistry and are specifically engineered to be rechargeable. While they share the “lithium” name, their internal chemistry and applications differ significantly.
Q:Are lithium batteries rechargeable?
A:Strictly speaking, standard lithium batteries are non-rechargeable (primary cells). Attempting to recharge a primary lithium battery can lead to overheating or fire. However, lithium-ion (Li-ion) and Lithium Iron Phosphate (LiFePO4) batteries are secondary cells designed for recharging. In common language, people often use “lithium battery” to refer to both, so it is vital to check the label for “rechargeable” before connecting to a charger.
Note:
[1]Understanding energy density is crucial for selecting the right battery for your needs.↪
[3]Discover how volumetric energy density affects battery design and application suitability.↪
[6]Learn about dendrite formation and its implications for battery safety and performance.↪
[7]Learn about the critical role of cell voltage in battery performance and safety.↪
[8]Get insights into the rigorous testing required for battery safety compliance.↪
[9]Understand the logistical issues surrounding battery replacement in hard-to-reach areas.↪
[10]Understand the impact of temperature on battery efficiency and safety.↪
Also read further information about lithium metal battery:
- The Ultimate Guide to Lithium Metal Battery for Professionals
- What Makes the Science Behind Lithium Thionyl Chloride Battery So Unique?
- The 3.0V Standard: Unlocking the Power of Lithium Manganese Dioxide Batteries
- Beyond 500 Wh/kg: Lithium Metal Battery Energy Density Under Real IoT Load Conditions
- The Science of the Plateau: Why Voltage Stability is the Lifeline of in Power System
- Operating Temperature Range: How temperature affects lithium metal battery performance
- The Real Cost of Cheap Batteries: How High Self Discharge Rate Increase Your TCO
- The Silent Shelf-Life Secret: Why Battery Passivation is Actually a Good Thing
- Mechanical Robustness: Designing Robust Battery That Survive Shock, Vibration, and Long-Term Stress
- Lithium Metal Batteries for Long-Life Industrial Applications: Design and Performance Insights
- Thermal Runaway Prevention: Why Factory-Level Testing is Non-Negotiable for Lithium Battery Safety
- Lithium Metal Battery Long Term Cost Analysis: Initial Price vs Lifetime Value
- Solid state batteries vs lithium metal batteries: Which One Elevates Your Product Performance?


